A sticky situation: How a molecular ‘adhesive’ may be the key to making an antibiotic more effective
October 17, 2025
Antibiotics are essential tools in modern medicine. They treat bacterial infections by killing harmful bacteria or making it harder for them to grow and multiply.
Whether it be for strep throat or wound infections, most of us have likely taken an antibiotic before. But their power comes with a caveat: some antibiotics can have unwanted side effects that also impact healthy cells.

“Antibiotics don’t all work the same — they each have their own unique plan of attack,” said Huilin Li, Ph.D., the Ralph and Grace Hauenstein Endowed Chair in Structural Biology at Van Andel Institute. “Understanding how antibiotics function at the cellular level can help us develop more effective, targeted medications with fewer side effects.”
One such antibiotic is neomycin, a medication commonly used as a topical treatment for bacterial skin infections. Neomycin works by blocking protein production, which is an essential process for bacterial survival. While effective for treating skin conditions, its broader clinical use as an oral antibiotic is limited because it can harm healthy cells in the kidneys and ears.
Scientists like Li and collaborator Todd Graham, Ph.D., of Vanderbilt University, are searching for new ways to improve these crucial medications while minimizing side effects. To this end, they’re exploring why some non-bacterial cells, such as those in the ears and kidneys, are so sensitive to neomycin. Their goal: understand how neomycin enters cells, and how we might control that process to reduce its side effects.
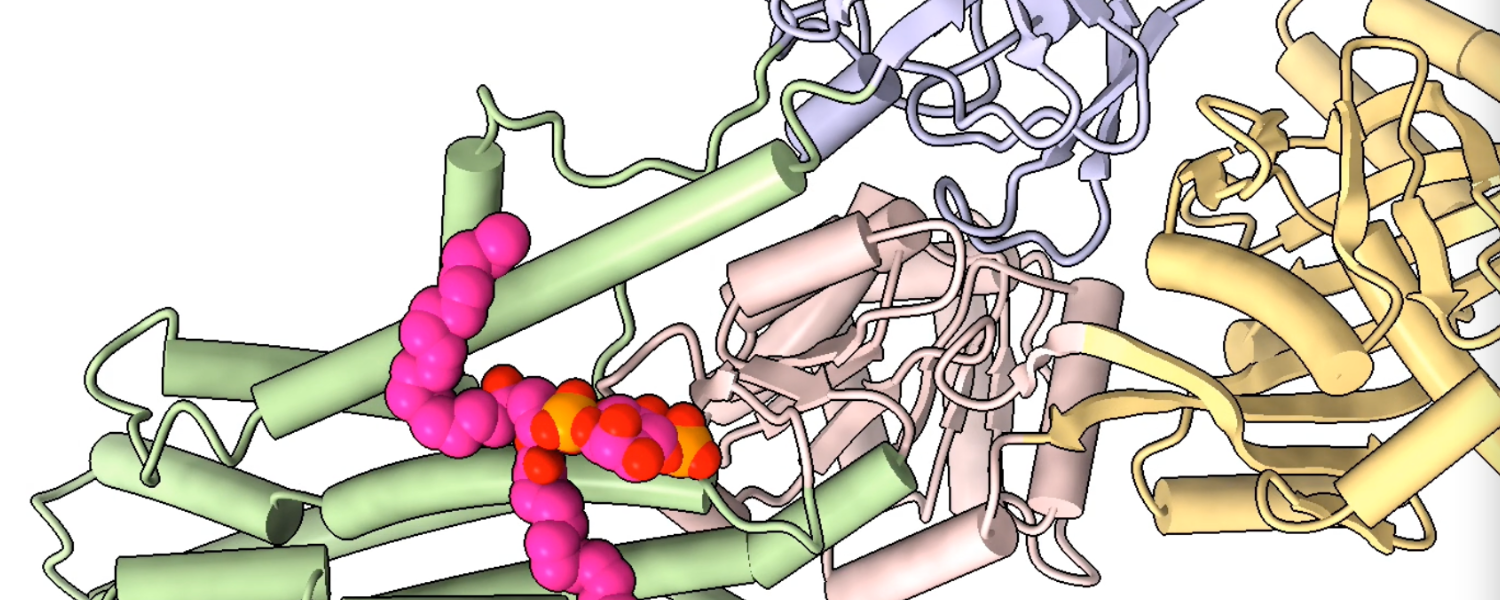
Solving a sticky situation
Neomycin sensitivity is linked to a protein called Neo1 in yeast and ATP9A in humans. ATP9A is found mostly in the brain and, to a smaller extent, the kidneys.
In a 2021 study, the Li Lab found that Neo1 acts as a lipid flippase, a specialized protein that transports specific lipid molecules from outside of the cell membrane to the inside. This important process helps ensure integrity of the cell membrane and enables cells to respond to shifts in their environments.
During this project, the team made an unexpected discovery, said study co-author Diessel Duan, Ph.D., a research scientist in the Li Lab. They found that a lipid signaling molecule called PI4P binds to Neo1, which activates its transport properties. At the time, Duan said, the reason behind the interaction was unclear.
That wasn’t the only mystery: they still didn’t understand exactly how neomycin could enter cells in the body.
But now, thanks to follow up work published this summer, we have answers.
In their new study, which appeared in the journal Nature Cell Biology, Li, Graham and collaborators found that Neo1 (and its human equivalent, ATP9A) actively transports PI4P to the inside of the cell membrane, preventing neomycin from entering the cell.
The team also tracked how PI4P moves inside the cell. They discovered that when Neo1 in yeast and ATP9A in humans are disrupted, PI4P is left exposed on the cell surface. The exposed PI4P functions like a molecular adhesive, attracting neomycin and allowing it to enter the cell, where it exerts its toxic effects.
The findings reveal how cells become sensitive to neomycin and how they manage lipid-signaling molecules. Together, these insights could help refine antibiotics like neomycin.
“Our work has important implications for understanding how molecules like PI4P may communicate on the cell surface,” said Duan. “Targeting these lipid molecules could be a game-changer in overcoming neomycin’s adverse effects.”
Funding Acknowledgment
Research reported in this publication was supported by Van Andel Institute; the National Institute of General Medical Sciences of the National Institutes of Health under award no. R35GM144123 (Graham) and the National Cancer Institute of the National Institutes of Health under award no. R01CA231466 (Li). Experiments were performed in part through the use of the Vanderbilt Cell Imaging Shared Resource, which is supported by the National Institutes of Health under award no. S10OD021630. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding organizations.



